Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Rizaal, M.; Nakajima, Kunihisa; Saito, Takumi*; Osaka, Masahiko; Okamoto, Koji*
ACS Omega (Internet), 7(33), p.29326 - 29336, 2022/08
Times Cited Count:3 Percentile:27.64(Chemistry, Multidisciplinary)Rizaal, M.; Nakajima, Kunihisa; Saito, Takumi*; Osaka, Masahiko; Okamoto, Koji*
Journal of Nuclear Science and Technology, 57(9), p.1062 - 1073, 2020/09
Times Cited Count:8 Percentile:70.65(Nuclear Science & Technology)The interaction of cesium hydroxide and a calcium silicate insulation material was experimentally investigated at high temperature conditions. A thermogravimetry equipped with differential thermal analysis was used to analyze thermal events in the samples of mixed calcium silicate and cesium hydroxide under Ar-5%H and Ar-4%H
and Ar-4%H -20%H
-20%H 0 with maximum temperature of 1100
0 with maximum temperature of 1100 C. Prior being mixed with cesium hydroxide, a part of calcium silicate was pretreated at high temperature to evaluate the effect of possible structural changes of this material due to a preceding thermal history and also the sake of thermodynamic evaluation to those available ones. Based upon the initial condition (preliminary heat treatment) of calcium silicate, it was found that if the original material consisted of xonotlite (Ca
C. Prior being mixed with cesium hydroxide, a part of calcium silicate was pretreated at high temperature to evaluate the effect of possible structural changes of this material due to a preceding thermal history and also the sake of thermodynamic evaluation to those available ones. Based upon the initial condition (preliminary heat treatment) of calcium silicate, it was found that if the original material consisted of xonotlite (Ca Si
Si 0
0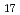 (0H)
(0H) ), the endothermic reaction with cesium hydroxide occurred over the temperature range 575-730
), the endothermic reaction with cesium hydroxide occurred over the temperature range 575-730 C meanwhile if the crystal phase of original material was changed to wollastonite (CaSi0
C meanwhile if the crystal phase of original material was changed to wollastonite (CaSi0 ), the interaction occurred over temperature range 700-1100
), the interaction occurred over temperature range 700-1100 C. Furthermore, the X-ray diffraction analyses have indicated on both type of pretreated calsils that regardless of Ar-5%H
C. Furthermore, the X-ray diffraction analyses have indicated on both type of pretreated calsils that regardless of Ar-5%H and Ar-4%H
and Ar-4%H -20%H
-20%H 0 atmosphere, cesium aluminum silicate, CsAlSi0
0 atmosphere, cesium aluminum silicate, CsAlSi0 was formed with aluminum in the samples as an impurity or adduct.
was formed with aluminum in the samples as an impurity or adduct.
Nakajima, Kunihisa; Nishioka, Shunichiro*; Suzuki, Eriko; Osaka, Masahiko
Mechanical Engineering Journal (Internet), 7(3), p.19-00564_1 - 19-00564_14, 2020/06
A large amount of cesium (Cs) chemisorbed onto stainless steel is predicted to be present especially in the upper region of reactor pressure vessel (RPV) during light water reactor severe accident (LWR SA) and a chemisorption model was developed for estimation of such amounts of Cs for stainless steel type 304 (SS304). However, this existing chemisorption model cannot accurately reproduce experimental results. Therefore, in this study, a modified Cs chemisorption model which accounts for silicon content in SS304 and concentration of cesium hydroxide (CsOH) in gaseous phases was constructed by combining penetration theory for gas-liquid mass transfer with chemical reaction and mass action law for CsOH decomposition at interface between gaseous and solid phases. As a result, it was found that the modified model was able to reproduce the experimental data more accurately than the existing model.
Nishioka, Shunichiro; Nakajima, Kunihisa; Suzuki, Eriko; Osaka, Masahiko
Journal of Nuclear Science and Technology, 56(11), p.988 - 995, 2019/11
Times Cited Count:12 Percentile:79.53(Nuclear Science & Technology)In order to contribute to improvement of Cs chemisorption model used in severe accident analysis codes, the influence of chemical factors (temperature, atmosphere, concentration of affecting chemical elements etc.) on the Cs chemisorption behaviour onto stainless steel was investigated experimentally. It was found that the surface reaction rate constant used in the current Cs-chemisorption model was influenced by not only temperature, as already known, but also atmosphere, cesium hydroxide (CsOH) concentration in the gas phase and silicon content in SS304. Such chemical factors should be considered for the construction of the improved Cs-chemisorption model. Another important finding is that the chemisorption behavior at lower temperatures, around 873 K, could differ from those above 1073 K. Namely, Cs-Fe-O compounds would form as the main Cs-chemisorbed compounds at 873 K while Cs-Si-Fe-O compounds at more than 1073 K.